Introduction
Day 15 absolute lymphocyte count (ALC-15) recovery after autologous peripheral blood hematopoietic stem cell transplantation (APBHSCT) is recognized as a prognostic factor for survival. ALC-15 recovery is directly dependent on the amount of collected and infused autograft-absolute lymphocyte count (A-ALC). Our group published a double-blind phase III study which showed that patients infused with an A-ALC ≥ 0.5 x 109 cells/kg experienced superior survival post-APBHSCT. Based on the results from our phase III study, on April 1, 2017, our Bone Marrow Transplant Program changed our standard clinical practice to collect an A-ALC ≥ 0.5 x 109 cells/kg in addition to stem cells for lymphoma patients undergoing APBHSCT. To continue to assess the prognostic ability of A-ALC, we evaluated the overall survival (OS) and progression-free survival (PFS) of diffuse large B-cell lymphoma (DLBCL) patients that underwent APBHSCT after April 1, 2017 compared with a matched-control group in a 1:1:1 ratio with DLBCL patients infused with an A-ALC < 0.5 x 109 cells/kg or an A-ALC ≥ 0.5 x 109 cells/kg prior to April 1, 2017.
Methods
Eight-five consecutive DLBCL patients after our practice changed on April 1, 2017 received an A-ALC ≥ 0.5 x 109 cells/kg. For each DLBCL patient infused with an A-ALC ≥ 0.5 x 109 cells/kg after April 1, 2017, the set of potential controls was restricted to those DLBCL patients infused with an A-ALC < 0.5 x 109 cells/kg and an A-ALC ≥ 0.5 x 109 cells/kg prior to April 1 2017 . Within this group of potential controls, the subjects were chosen who best matched for the cases in terms of clinical risk variables, including gender, age, stage, lactate dehydrogenase (LDH), performance status, extra-nodal disease, International prognostic index (IPI), and disease status prior to APBHSCT (complete or partial response), using the GREEDY algorithm. Cases were defined as DLBCL patients infused with an A-ALC ≥ 0.5 x 109 cells/kg starting from April 1 2017. Control 1 was defined as DLBCL patients infused with an A-ALC < 0.5 x 109 cells/kg prior to April 1 2017. Control 2 was defined as DLBCL patients infused with an A-ALC ≥ 0.5 x 109 cells/kg prior to April 1 2017. We truncated the survival follow-up to 3 years starting from the date of transplant to match follow-up time between cases and controls. Both matched cases and control groups patients received BEAM conditioning. This retrospective study was approved by the Mayo Clinic Institutional Review Board according to the regulation of the Declaration of Helsinki.
Results
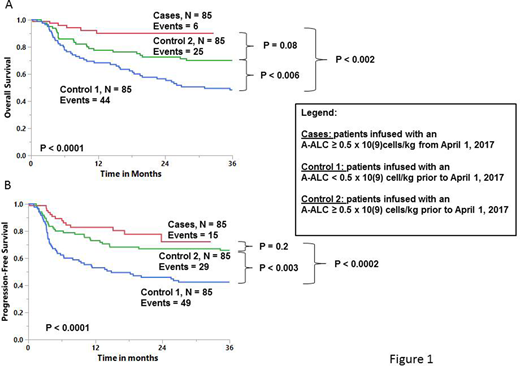
Cases, control 1 and control 2 were balanced to the clinical risk variables; age (p = 0.8); gender (p = 0.9); LDH (p = 0.6); stage (p = 0.3); performance status (p = 0.5); extra-nodal disease (p = 0.2); IPI (p = 0.6); and clinical status prior to transplant (p = 0.2). Figure 1A showed the OS and figure 1B the PFS. Both the cases and control 2 showed superior OS and PFS in comparison to control 1. Cases and control 2 showed overall similar OS and PFS. Multivariate analysis including all patients continue to depict the A-ALC ≥ 0.5 x 109 cells/kg as an independent predictor for OS (HR = 0.382, 95%CI=0.241-0.605, p < 0.0001) and PFS (HR = 0.437, 95%CI = 0.279-0.629, p <0.0001). Subgroup analysis, including all patients, shows superior OS and PFS for patients infused with an A-ALC ≥ 0.5 x 109 cells/kg for each of the following clinical variables: age [> 60 years: OS (p <0.0001); PFS (p <0.0001) or ≤ 60 years: OS (p <0.01); PFS (p , 0.02)]; extra-nodal disease [> 1: OS (p <0.008); PFS (p <0.01) or ≤1: OS (p < 0.001): PFS (p <0.0005)]; IPI [> 2: OS (p <0.01); PFS (p <0.02) or ≤ 2: OS (p <0.0008); PFS (p <0.0006)]; LDH [abnormal: OS (p <0.006); PFS (p <0.005) or normal: OS (p <0.002); PFS (p <0.002)]; performance status [> 1: OS (p <0.04); PFS (p <0.03) or ≤ 1: OS (p <0.0001); PFS (p <0.0001)]; stage [I/II: OS (p <0.006); PFS (p <0.006) or III/IV: OS (p <0.002); PFS (p <0.001)]; response prior to transplant [complete response: OS (p <0.0007); PFS (p <0.0001) or partial response: OS (p <0.01); PFS (p <0.02)].
Conclusion
Our match-control study continues to support the results of our Phase III study showing that the infusion of A-ALC is a prognostic factor for survival of DLBCL patients undergoing APBHSCT. Our studies support the practice of not only collecting enough stem cells (i.e., CD34) for hematologic engraftment, but also enough immune effector cells (i.e., A-ALC) to improve clinical outcomes in DLBCL patients post-APBHSCT.
Ansell:Regeneron: Research Funding; Bristol Myers Squibb: Research Funding; Trillium: Research Funding; ADC Therapeutics: Research Funding; Takeda: Research Funding; AI Therapeutics: Research Funding; Affimed: Research Funding; Seattle Genetics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal